TOTIM have been proven to reduce preparation time, especially for difficult set-ups
Reducing rates of misalignments reduces the number of repeated port films, saving therapists and physicians valuable time


































UNIQUE FEATURE
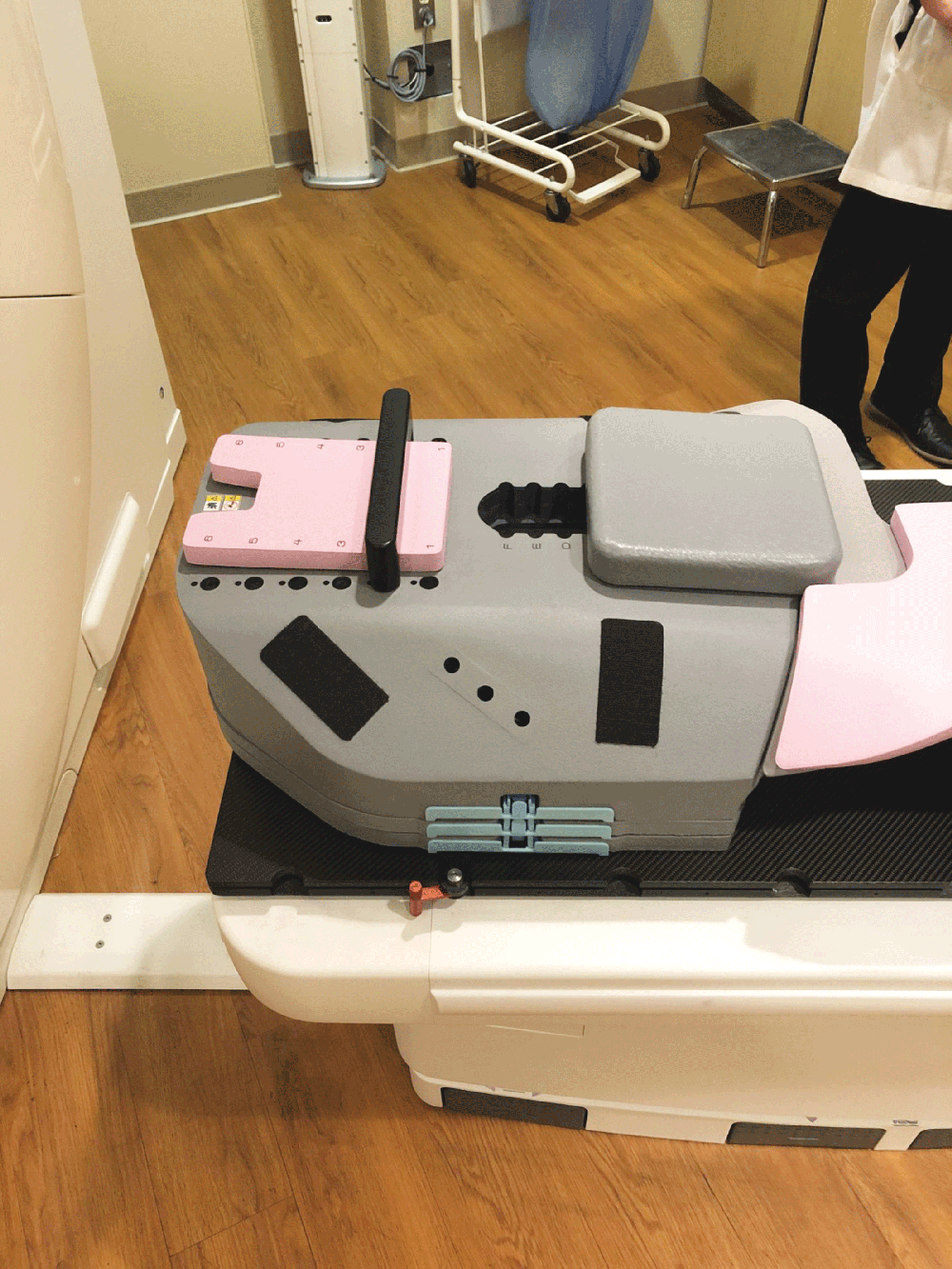
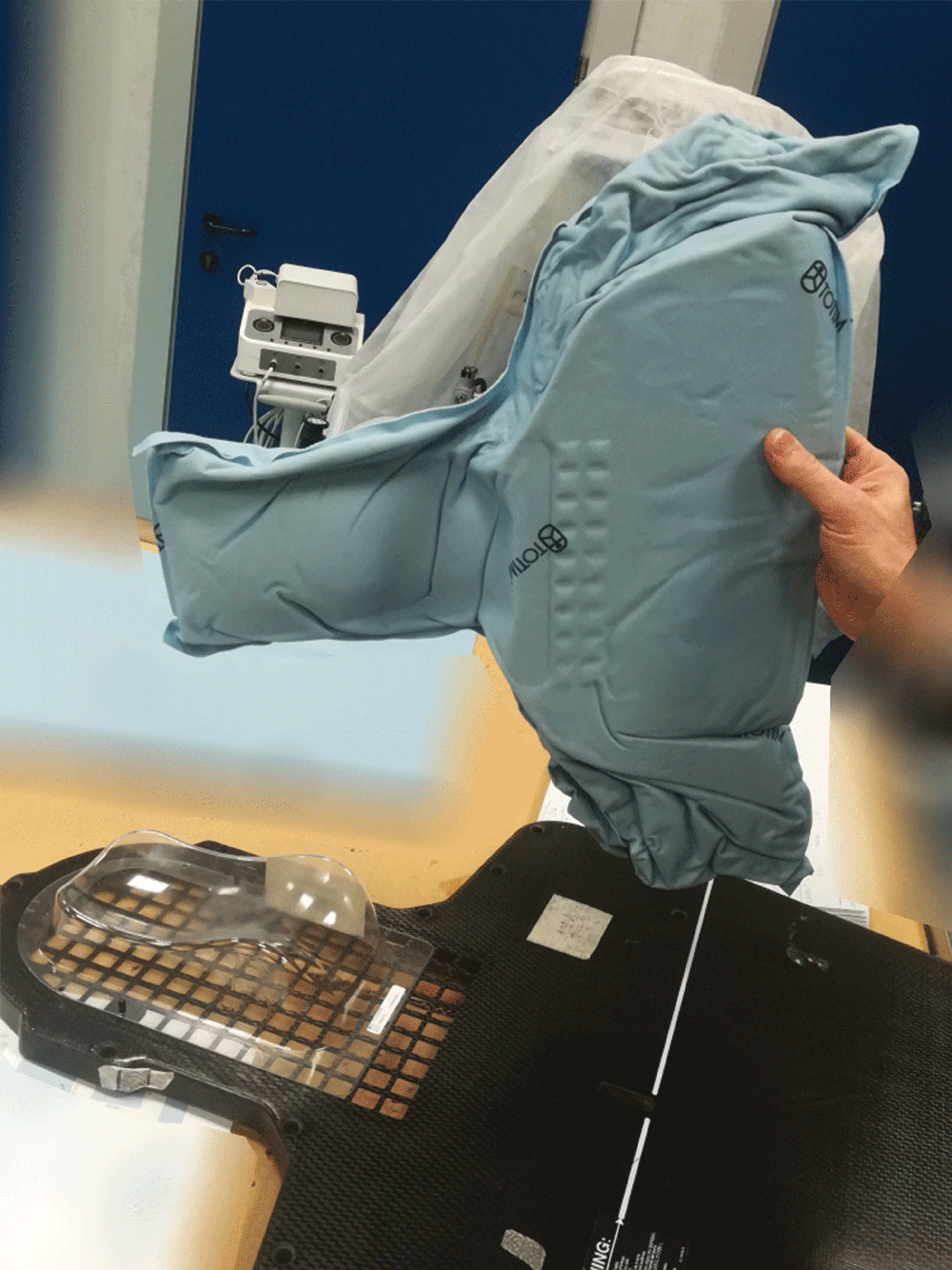
1. Fully sealed and expanding foam cushion
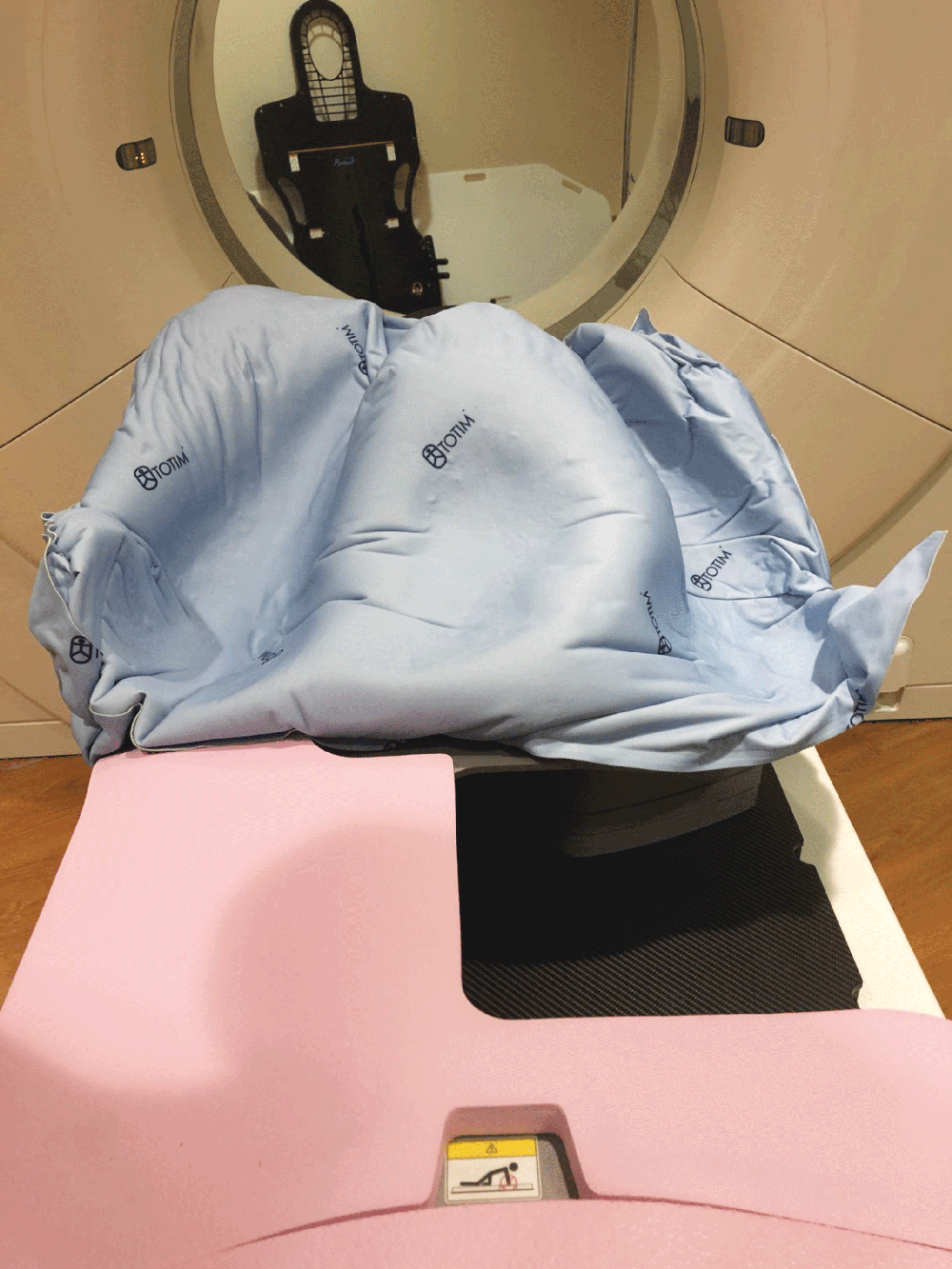
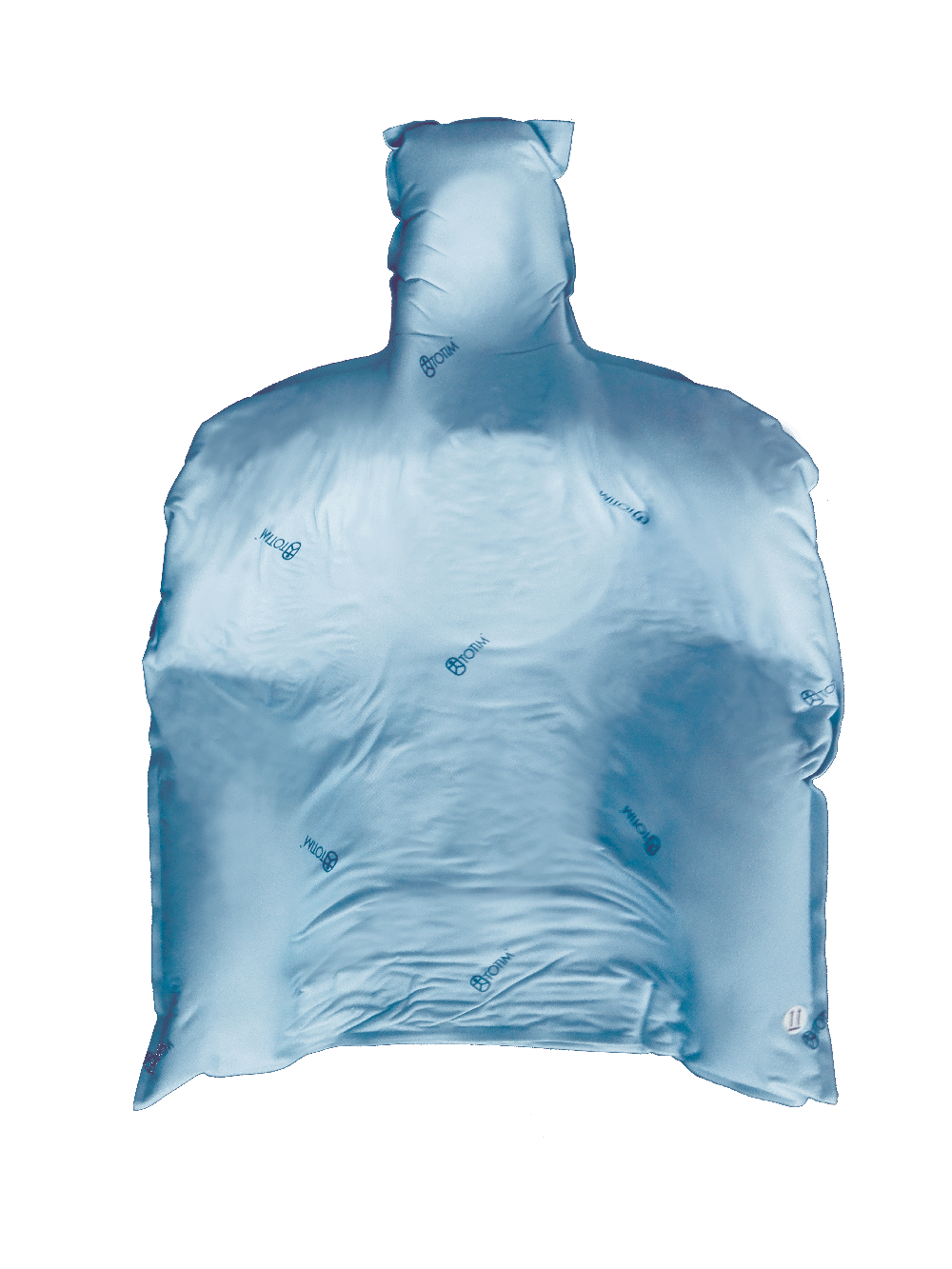
Totim is an expanding foam cushion, fully sealed with an outer microfiber polyester cover.
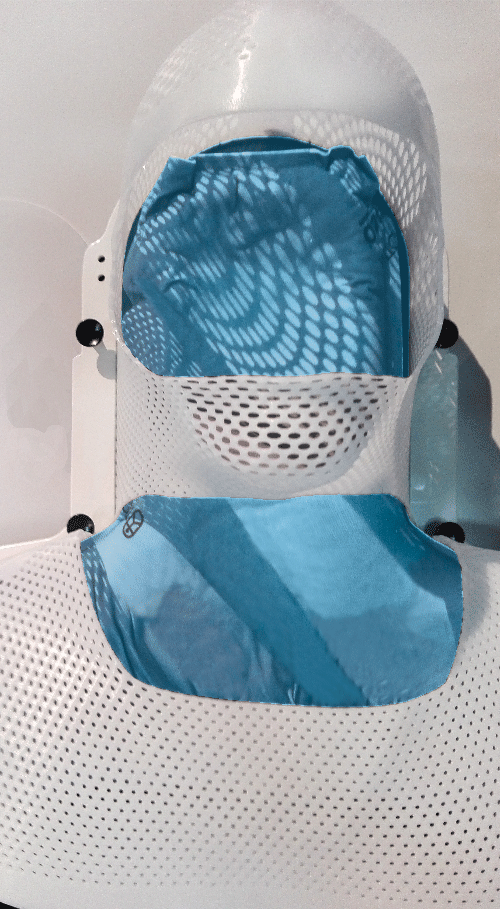
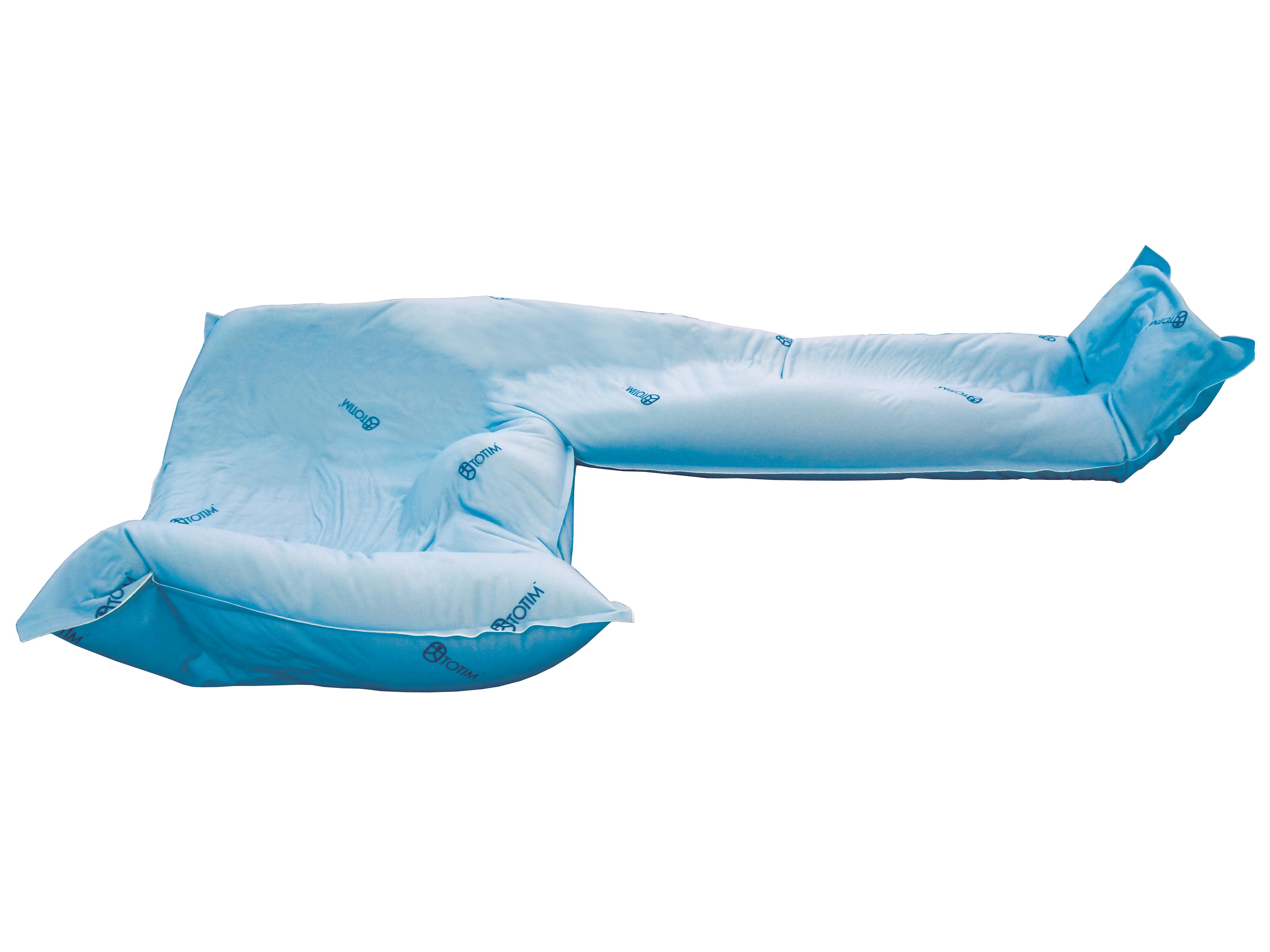
2. From extremity to whole body
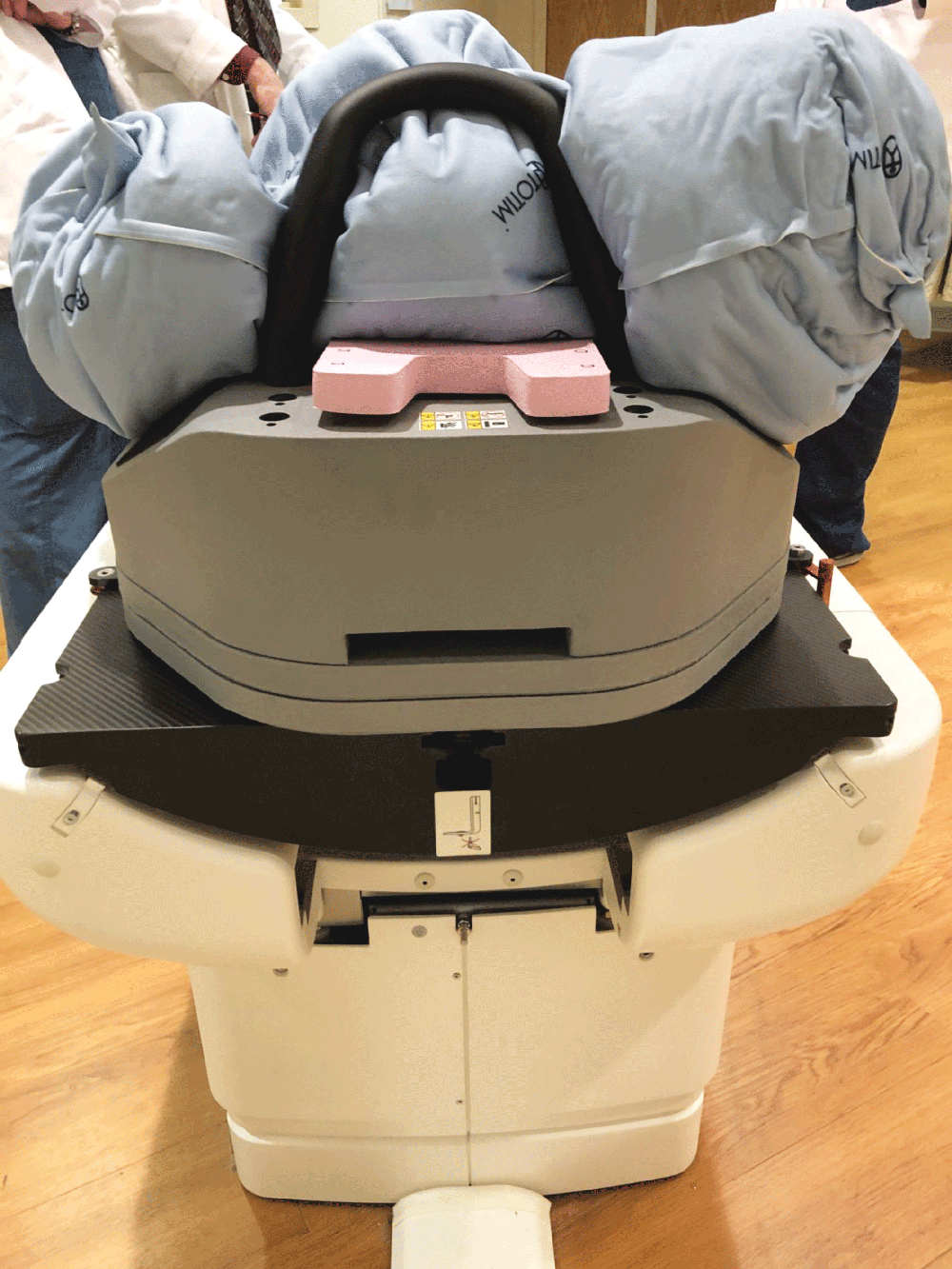
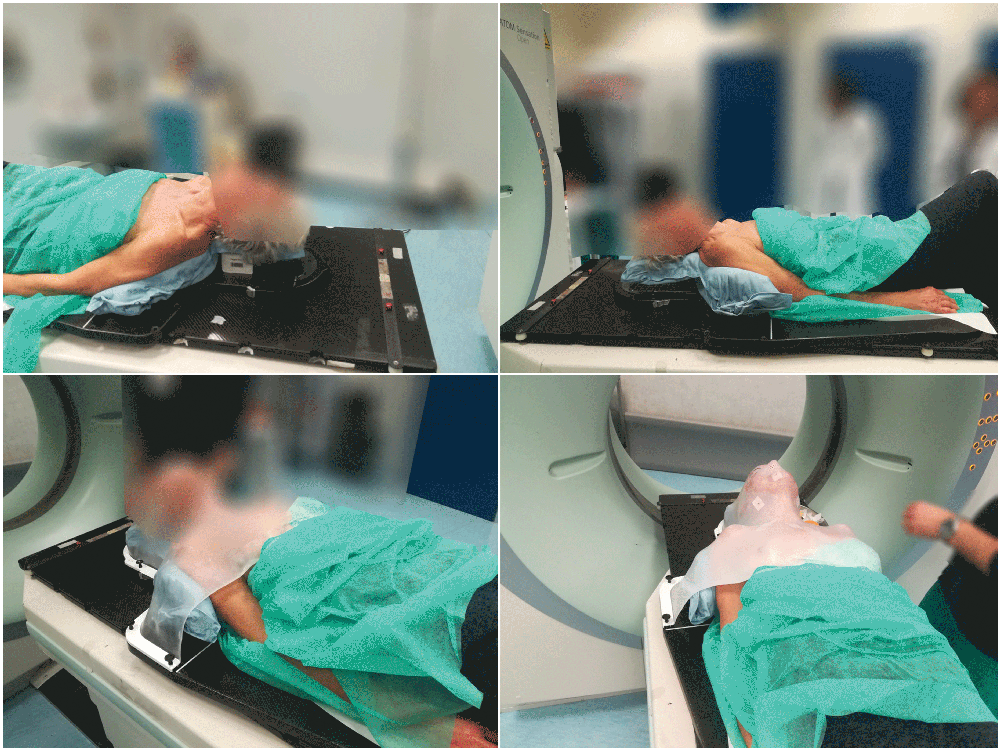
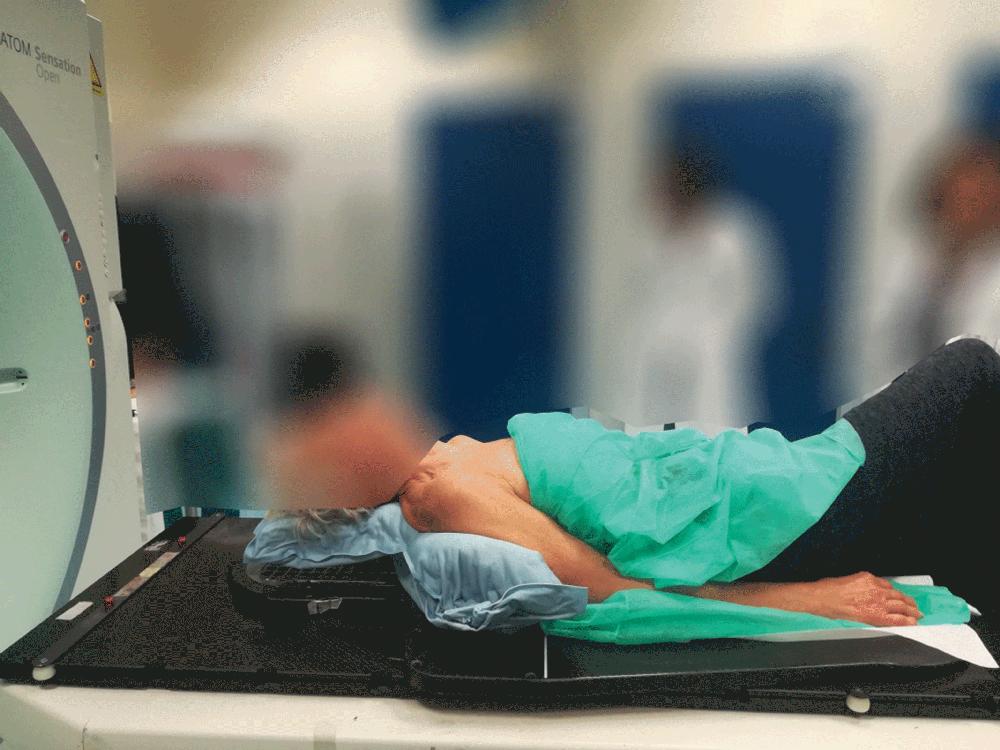
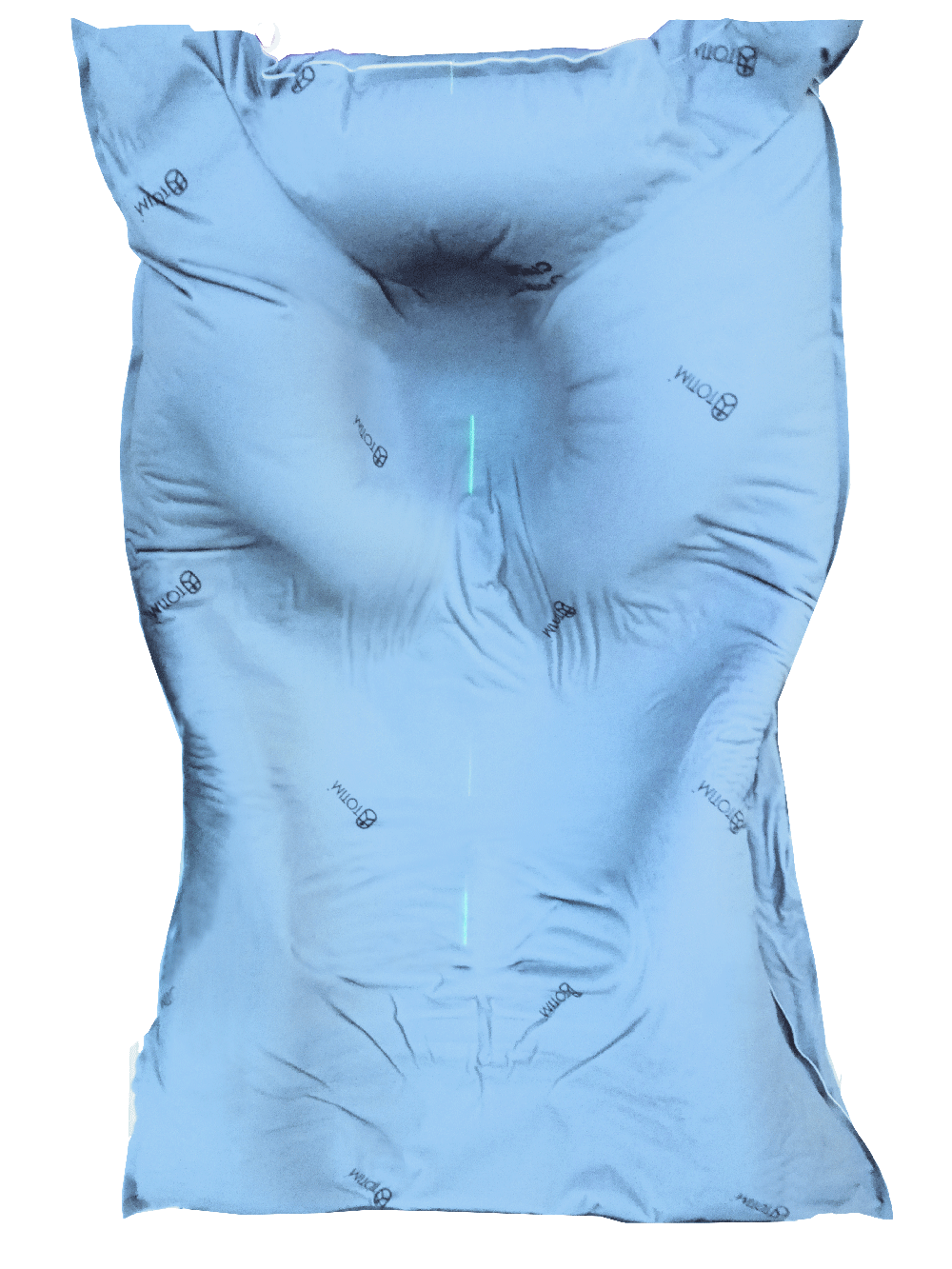
Totim cushions are specifically designed for many different parts of the body, from individual limbs to whole body.
3. No air or moisture activation
Totim cushion form by activation of internal component to then shape and mold around the patient. Does not require vacuum or water activation process to form rigid cushion.
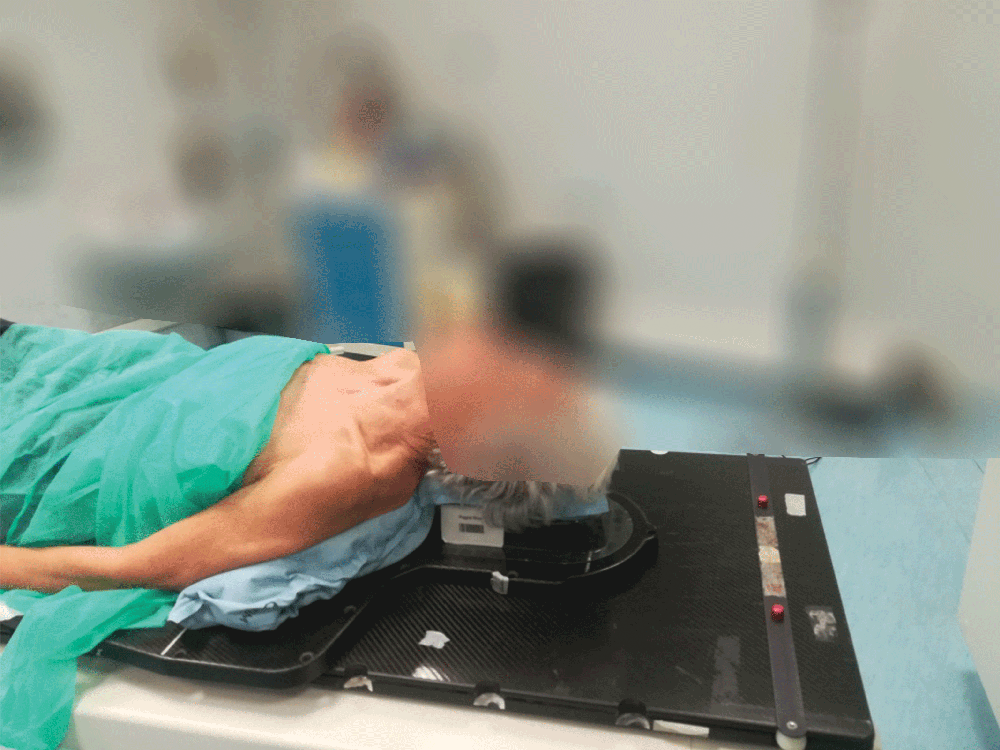
4. Just hold to mold
A simple process including activating the cushion contents, positioning the patient and then holding the cushion’s position. The expanding foam will then mold around the patient.
5. Durable
The patient can get in and out of a properly made form all day long without damaging it or changing its shape.
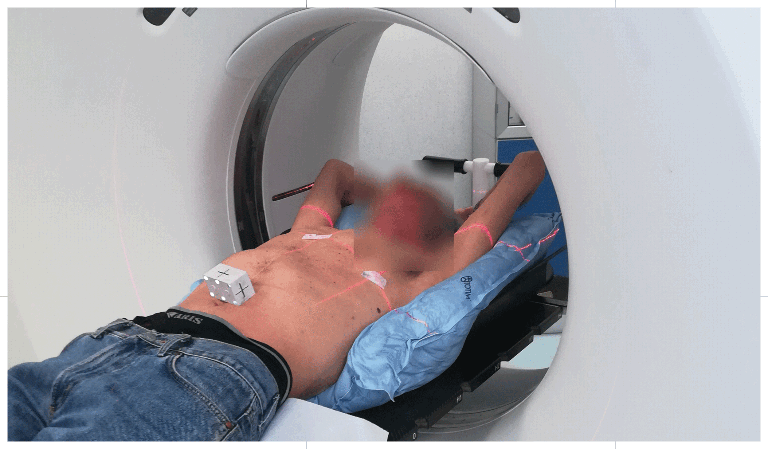
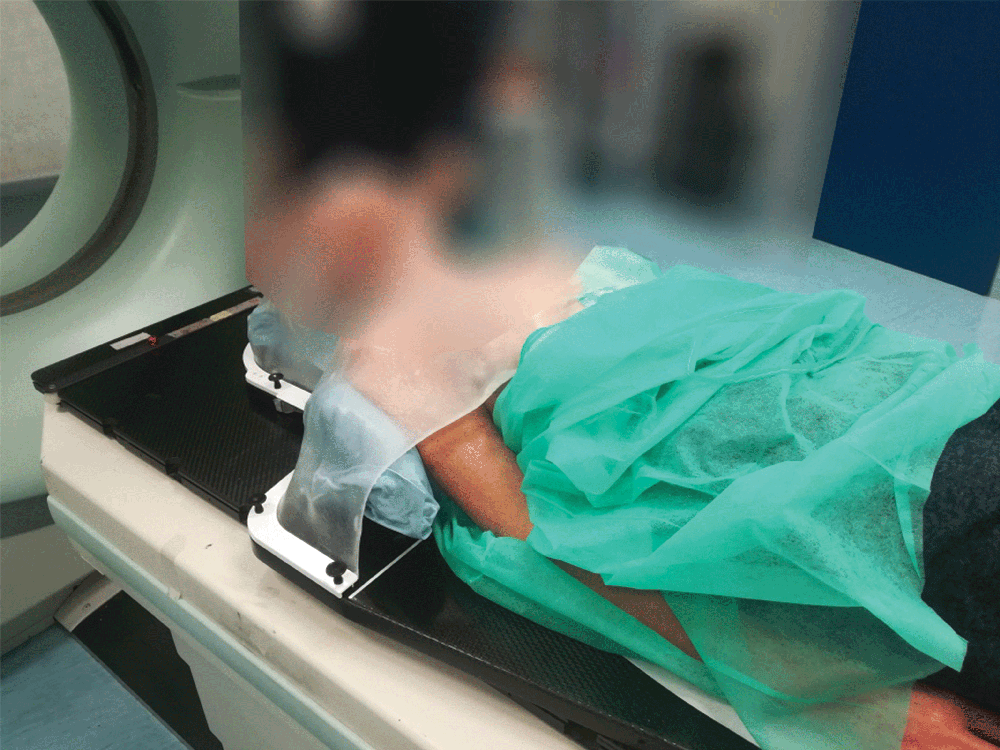
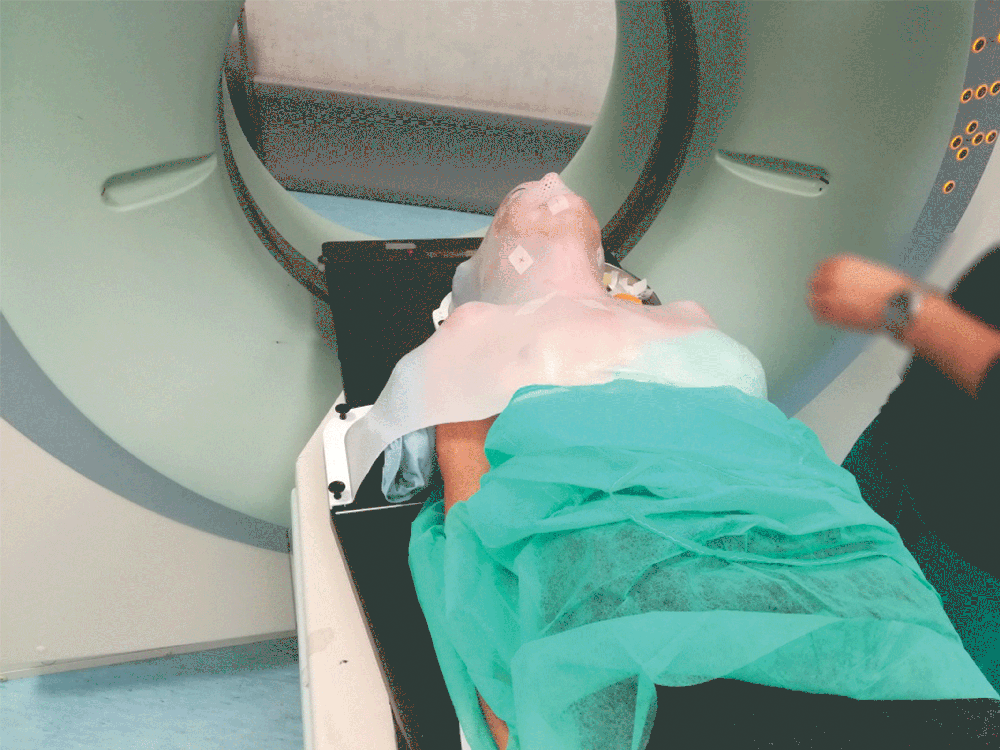
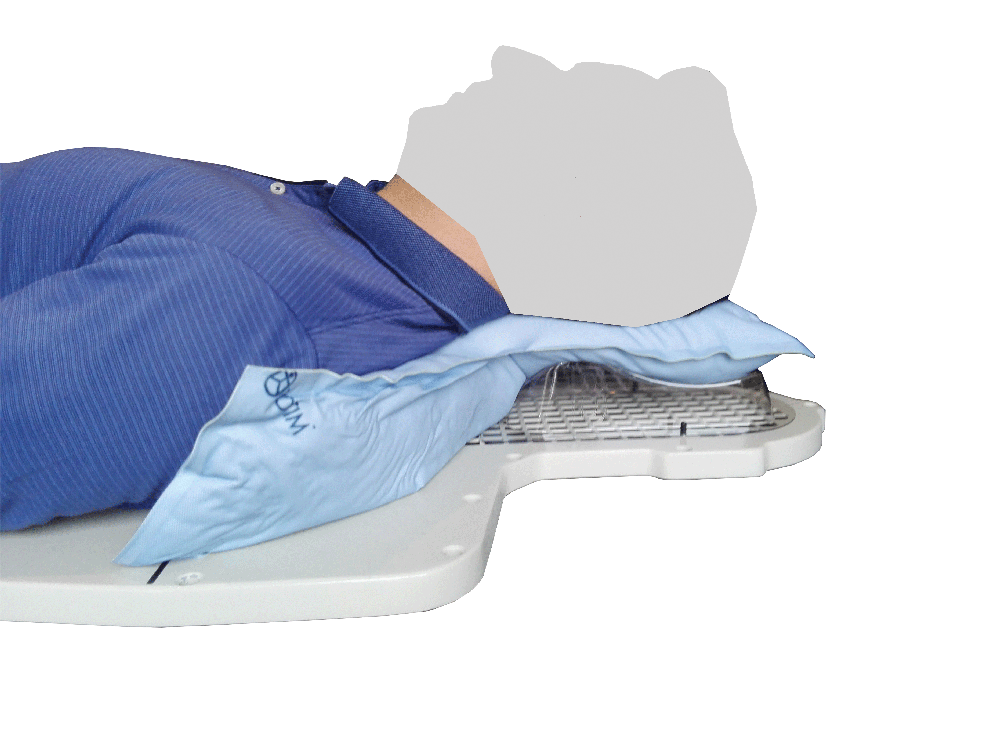
Setting up the patient in a reproducible position for accurate daily treatment.
TOTIM® is a fully sealed polyurethane foam cushion covered with microfiber polyester tissue for patient positioning and immobilization during simulation, planning and radiation treatment. The cushion shapes and molds around the patient after activation of the internal components. It maintains its formed shape during all sessions of radiotherapy treatment, enabling reproducibility of patient position.
Features & Clinical Benefits
TOTIM® Patient Cushions Immobilization System is indicated to position and/or immobilize and/or repositioning forms both adult and pediatric patients undergoing radiation therapy of the body, head, brain, neck, cervical, arm, limb, hand and leg including Stereotactic Radiosurgery (SRS), Stereotactic Radiotherapy (SRT), Surface Guided Radiation Therapy (SGRT), and electron, photon and proton treatments. The device is also used during image acquisition, including Computed Tomography (CT) and Magnetic Resonance (MR) Imaging, to support treatment planning.
Technical Characteristics:
Cushion with external cover in microfiber comfortable and absorbent water repellent non-woven material, markable, washable/easily sanitized, temperature controlled exothermic reaction, the short window of time required for solidification allows for the correct modeling of the external cover in microfiber polyester tissue fully sealed.
its formed shape during all sessions of radiotherapy treatment, enabling reproducibility of patient position.
TOTIM® is a polyurethane foam immobilization system using fully sealed bag/cushion.
The device can be attached to the treatment or simulation couch, extension, or overlay along with other optional positioning and immobilization devices and accessories.
Materials: Cushion in Microfiber Polyester / PU (fully sealed) and internal pouch (fully sealed) with inside the two reagents ready to be mixed.
Non-clinical testing was completed to confirm that TOTIM is safe and effective device.
A scientific rationale was used to address RF heating, magnetically induced torque, and magnetically induced displacement force.
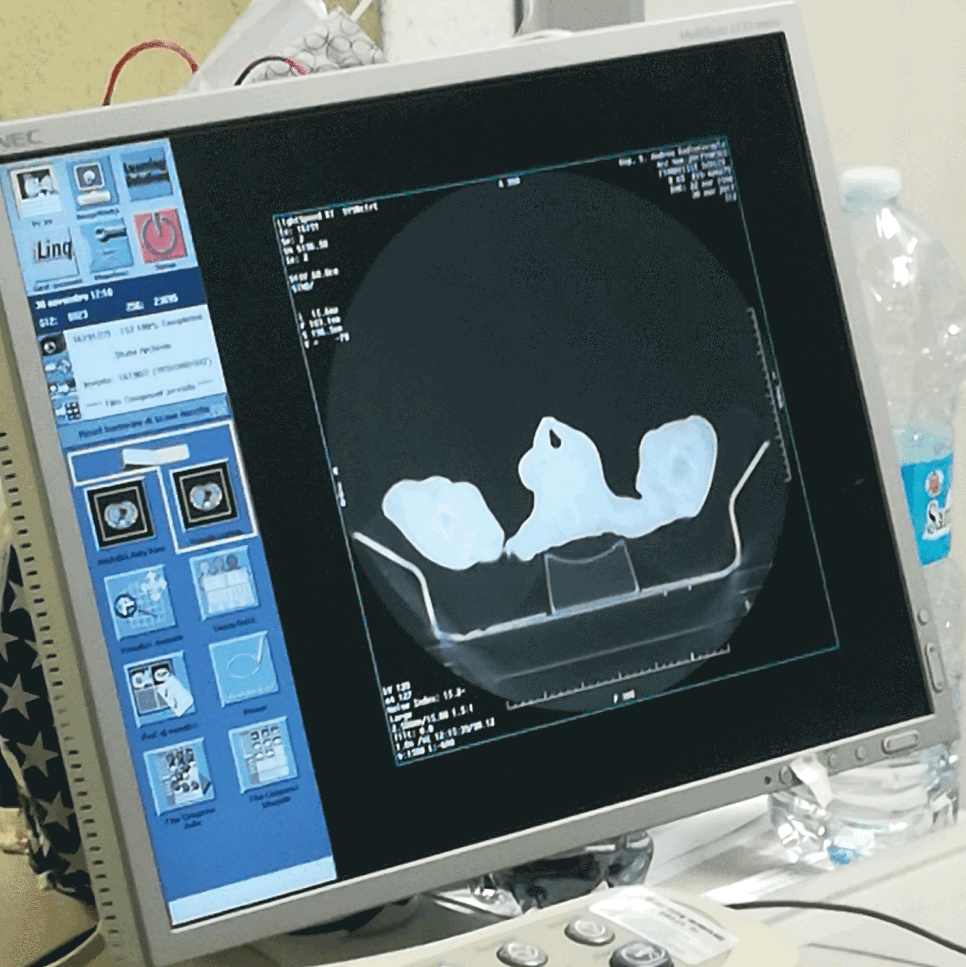
The TOTIM® device was tested with ASTM F2119-07 (Reapproved 2013) Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants (Materials), TOTIM is MR Safe.
Proton Test Water Equivalent Thickness (WET) Values:
to verify that the product to not negatively impact a proton team when measured orthogonally through the permissible radiation area.
Conclusion: the product tested do not perturb or impact the proton beam in a way that cannot be accounted for in the proton treatment planning process.
- The devices are intended for limited contact duration (<24 hours) for surface devices (skin).
Biocompatibility: ISO 10993-5:2009, 10993-12: 2012 and ISO 10993-10: 2010.
The TOTIM Patient Cushions is manufactured of non-magnetic materials, MR SAFE
ESSEBI MEDICAL SRL TOTIM®
Strada Campo del Fiume 84
47896 Faetano, RSM
Phone: +378 0549 963858
Patent SM-P-201700411
DESIGNED IN ITALY
Made in RSM
Copyright 2024 TOTIM® - ESSEBI MEDICAL, All Rights Reserved