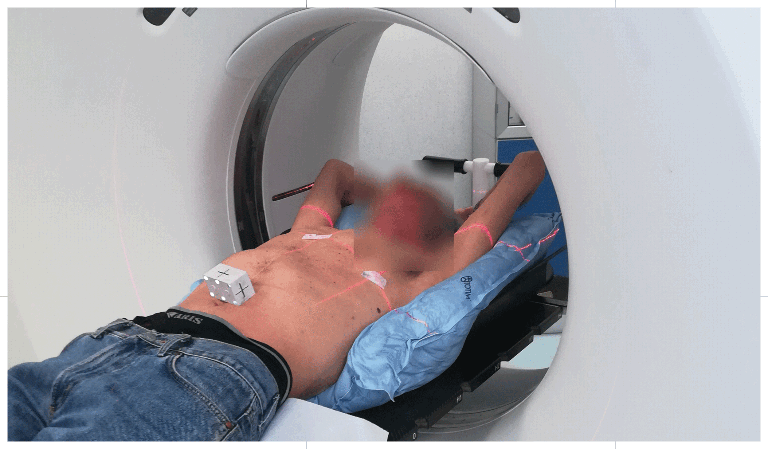











Device- Specific Intended Use:
The device maintains its formed shape during all sessions of radiotherapy treatment, enabling reproducibility of patient position. The device is molded at the back of the patient’s body, parts of the body or the head in order to hinder the usual range of movement occurring with non-customizable supports.
TOTIM is a polyurethane foam immobilization system using fully sealed bag/cushion.
The device can be attached to the treatment or simulation couch, extension, or overlay along with other optional positioning and immobilization devices and accessories.
Materials: Cushion in Microfiber Polyester / PU (fully sealed) and internal pouch (fully sealed) with inside the two reagents ready to be mixed.
Radiotherapy and Oncology, April 2018
https://www.researchgate.net/publication/325840871_EP-1961_Classical_Kaposi's_sarcoma_treatment_with...
Physics Medica, December 2018
https://www.sciencedirect.com/science/article/abs/pii/S1120179718302709/product/index
Non-clinical testing was completed to confirm that TOTIM is safe and effective device.
A scientific rationale was used to address RF heating, magnetically induced torque, and magnetically induced displacement force.
- The TOTIM device was tested with ASTM F2119-07 (Reapproved 2013) Standard Test Method for Evaluation of MR Image Artifacts from Passive Implants (Materials), TOTIM is MR Safe.
Proton Test Water Equivalent Thickness (WET) Values:
to verify that the product to not negatively impact a proton team when measured orthogonally through the permissible radiation area.
Conclusion: the product tested do not perturb or impact the proton beam in a way that cannot be accounted for in the proton treatment planning process.
- The devices are intended for limited contact duration (<24 hours) for surface devices (skin).
Biocompatibility:
ISO 10993-5:2009, 10993-12: 2012 and ISO 10993-10: 2010.
- Declaration of Conformity 93/42/CEE Medical Device Class I
- FDA 510(K) Cleared Device Class II
The device is used in a healthcare facility / hospital. The device is intended to be used on adult and pediatric patients.
Features & Clinical Benefits
- Disposable, easy to sanitize, markable and radiotransparent
- Pediatric options available
- MR Safe.
- The TOTIM Patient Cushions is manufactured of non-magnetic materials.
HEAD & NECK
The device is molded at the back of the patient’s body, parts of the head or the neck in order to hinder the usual range of movement occurring with non-customizable supports.
TOTIM IS PRODUCED IN VARIOUS SIZE AND CAN BE CUSTOMIZED TO MEET SPECIFIC DEMAND
TOTIM DEVICE IS PACKED IN SINGLE BOXES FOR SAFE AND PROTECTED SHIPPING
TOTIM GUARANTEES COMPLETE SAFETY AGAINST LEAKAGE OF THE BICOMPONENT THANKS TO THE RESEARCH DONE BY THE R&D SECTION OF ESSEBI MEDICAL